Work package number 5: INTENSIVE CARE & DONOR TRANSPLANT COORDINATION COLLABORATION
Leader: Department of Health – Organ and Tissue Transplantation – NHS (NHSBT), United Kingdom
PRODUCTS
-
Recommendations for improvement and toolkit methodology systemic Part 2
-
ACCORD WP5 ICU & DTC Collaboration FINAL REPORT
Objectives:
The aim of this work package (WP) is to describe the usual pathways applied to patients with a devastating brain injury and to explore their impact on the potential of donation and on the realization of the deceased donation process across European Union countries. This WP also aims at developing and proving by implementation an acceptable and effective rapid improvement toolkit that supports modifications in end-of-life management that promote donation, adapted to each identified end-of-life care model.
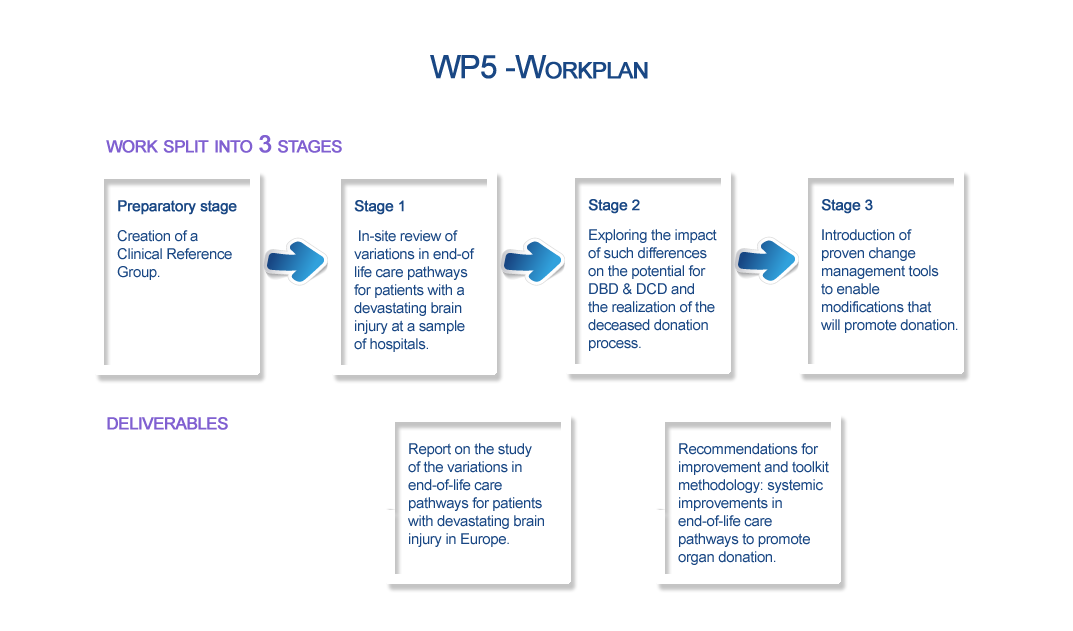
Activities:
Preparatory Stage: Creation of a Clinical Reference Group to design and facilitate the implementation of the subsequent activities.
Stage 1: In-site review of variations in end-of-life care pathways for patients presenting with a devastating brain injury at a sample of hospitals from participating Member States.
Stage 2: The impact of any such differences in end-of-life care on the potential for donation after brain death and after circulatory death, and on the realization of the deceased donation process will be explored. This will subsequently lead to identifying barriers to donation, exploring the most effective time/means of cooperation and consultation between clinicians and donor transplant coordinators and to agreeing on specific adaptations to facilitate donation in each pathway.
Stage 3: Proven change management tools will be introduced to enable modifications that will promote donation within existing pathways at participating countries. This will include training an expert for each associated partner, supporting clinicians to identify achievable interventions and use the methodology to change, supporting participating countries to implement change in selected hospitals, monitoring and evaluating effectiveness of changes, agreeing modifications to changes in care practice to be extended and writing up a methodology for further implementation in each European Union country.
Deliverables:
Deliverable 7: Report on the study of the variations in end-of-life care pathways for patients with devastating brain injury in Europe - Report describing end-of-life-practices for patients with a devastating brain injury and their impact on the potential of deceased donation and the realization of the deceased donation process in the European Union, based on a dedicated study on a sample of hospitals.
Deliverable 8: Recommendations for improvement and toolkit methodology: systemic improvements in end-of-life care pathways to promote organ donation - Report providing recommendations to be applied to end-of-life care pathways for promoting organ donation.
Associated Partners:
- CROACIA - Ministry of Health and Social Welfare of the Republic of Croatia. Department for Special Health Care and Transplantation (MOHSW).
- ESTONIA - Tartu University Hospital (TUH).
- FRANCE - Agence de la Biomédecine (ABM).
- GERMANY - Deutsche Stiftung Organtransplantation (DSO).
- GREECE - Hellenic Transplant Organistion (HTO).
- HUNGARY - Hungarian National Blood Transfusion Service (HNBTS).
- IRELAND - Health Service Executive (HSE).
- ITALY - Centro Nazionali Trapianti (CNT).
- LATVIA - Pauls Stradins Clinical University Hospital.
- LITHUANIA - National Transplant Bureau (NTB).
- PORTUGAL - The Authority of Blood Services and Transplantation (ASST).
- SLOVENIA - Slovenija Transplant.
- SPAIN - Organización Nacional de Trasplantes (ONT).
- THE NETHERLANDS - Nederlandse Transplantatie Stichting- Dutch Transplantation Foundation (DTF)
- UNITED KINGDOM - Department of Health, Organ and Tissue Transplantation (NHSBT).
Collaborating Partners:
- European Hospital and Healthcare Federation (HOPE).
- European Society of Intensive Care Medicine (ESICM).
- European Transplant Coordinators Organisation – European Donation Committee (ETCO-EDC), a section of ESOT.