Work package number 4: LIVING DONOR REGISTRIES
Leader: Nederlandse Transplantatie Stichting (Dutch Transplantation Foundation) (DTF), The Netherlands
PRODUCTS
-
Experience with Living Donation and Living Donor registries
-
ACCORD WP 4 FINAL Data set and data dictionary
-
ACCORD WP 4 Technical, organisational and governace requirements
-
ACCORD WP 4 Living Donor Registries FINAL REPORT
Objectives:
The aim of this work package (WP) is to improve Member States information systems on live organ donation through the provision of recommendations for the design and management of structured live donor registries and through setting down a model for supranational data sharing.
Activities:
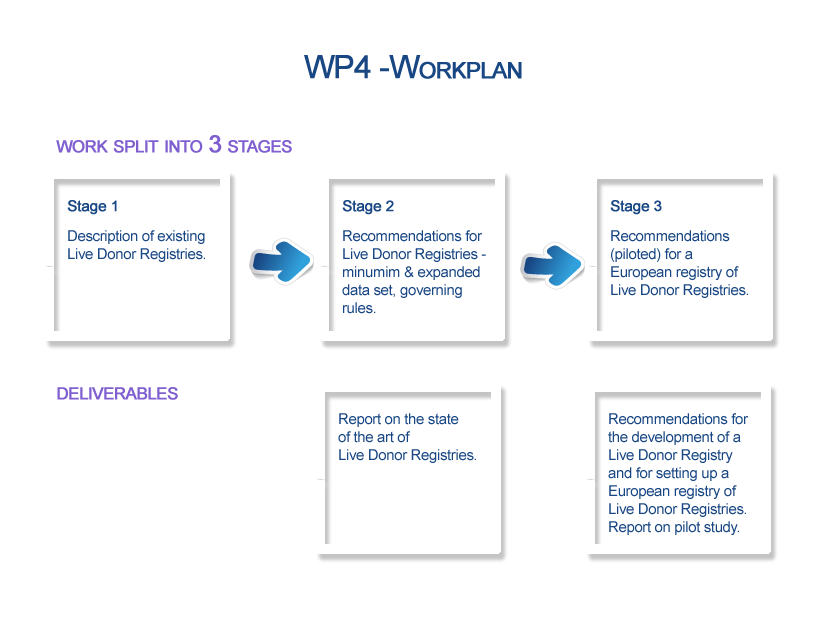
Stage 1: A description of existing national and international live donor registries will be carried out, including information on the collected variables (with definitions and coding) and on the governing, operational and technical rules applied.
Stage 2: Establishment of recommendations for developing a national live donor registry, defining a minimum and an expanded data set of variables, elaborating a data dictionary for variables in the minimum data set and specifying governing, operational, and technical rules. The minimum data set will include at least baseline information on donor demographics (including residence), relationship between donor and recipient, and clinical situation as well as that on complications related to donation that might appear in the short, mid and long-term.
Stage 3: Development of a model for international data sharing through setting the basis for a European registry of live donor registries. An analysis of the legal constraints and requirements, the development of a protocol for a registry of registries, including data modelling as well as governing, operational and technical requirements will be performed. A pilot of the registry of registries with 2-4 participating countries will be carried out.
Deliverables:
Deliverable 5: Report on the State of the art of live donor registries - Report compiling information on live donor registries currently in place, either national or international, including details on variables and definitions and governing, operational and technical rules applied.
Deliverable 6: Recommendations for the development of a live donor registry and for setting up a European Union registry of live donor registries. Report on the pilot study - Report providing recommendations for developing a live donor registry in terms of design and rules. Separate report with recommendations for international data sharing, based on the concept of a registry of live donor registries. Description of pilot action
Associated Partners:
- CROACIA - Ministry of Health and Social Welfare of the Republic of Croatia. Department for Special Health Care and Transplantation (MOHSW).
- FRANCE - Agence de la Biomédecine (ABM).
- GERMANY - Deutsche Stiftung Organtransplantation (DSO).
- IRELAND - Health Service Executive (HSE).
- ITALY – Centro Nazionali Trapianti (CNT).
- LATVIA - Pauls Stradins Clinical University Hospital.
- LITHUANIA - National Transplant Bureau (NTB).
- NORWAY - The Norwegian Directorate of Health (HDIR).
- POLAND - Poltransplant.
- PORTUGAL - The Authority of Blood Services and Transplantation (ASST).
- ROMANIA - National Transplant Agency (ANT).
- SLOVAK REPUBLIC – National Transplant Organisation (NTO).
- SPAIN - Organización Nacional de Trasplantes (ONT).
- THE NETHERLANDS - Nederlandse Transplantatie Stichting- Dutch Transplantation Foundation (DTF).
- UNITED KINGDOM - Department of Health, Organ and Tissue Transplantation (NHSBT).
Collaborating Partners:
- Eurotransplant.
- Scandiatransplant.
- Hospital Clínic, Barcelona (Spain).
- University Clinic of Gent (Belgium).