Work package number 6: TWINNING ON ORGAN DONATION AND TRANSPLANTATION
Leader: Agence de la Biomédecine (ABM), France.
Products:
Report on experiences with Twinning Projects in ACCORD
Guidelines for Twinning Activities on Donation and Transplantation practices in EU
Objectives:
WP 6 aims at implementing practical collaborations between European Union (EU) countries for the transfer of knowledge, expertise or tools in specific areas related to Directive 2010/53/EU and the Action Plan on organ donation and transplantation, based on comprehensive and specifically prepared protocols. This WP also aims at providing recommendations for future twinning initiatives in the field of organ donation and transplantation.
Activities:
- Preparatory stage:Selection and preparation of twinning projects to be carried out during ACCORD lifetime, based on a set of pre-specified criteria. Accepted proposals include:
- France supporting Bulgaria on structuring the organ procurement system at a national and regional level, improving the monitoring and evaluation system on organ procurement and transplantation activities and setting an operational and sustainable paediatric kidney transplantation programme;
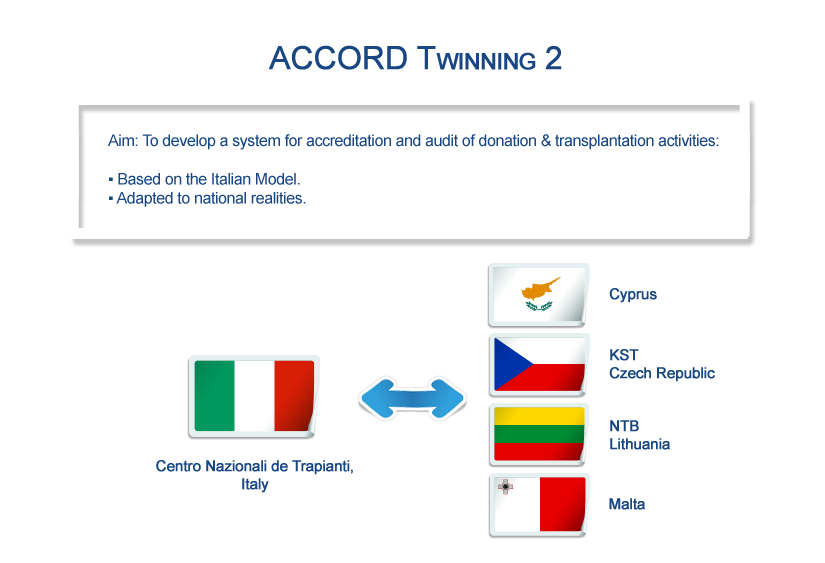
- Italy supporting Cyprus, Czech Republic, Lithuania and Malta on setting up a national accreditation system for transplantation centres.
- The Netherlands supporting Hungary on providing a number of surgeons with specific training on organ recovery.
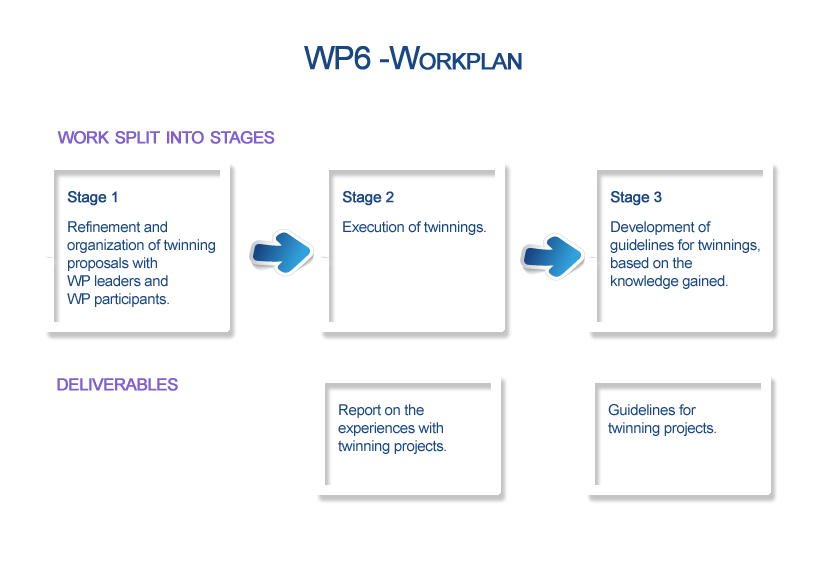
- Stage 1: Refinement and organization of twinning proposals.
- Stage 2: Execution of twinnings with a close follow-up of collaborations ensured by continuous reporting, evaluation and input from other participants. Each twinning will be comprehensively described, enclosing results achieved.
- Stage 3: Development of guidelines for twinning, based on the knowledge gained.
Deliverables:
- Deliverable 9: Report on experiences with Twinning Projects in ACCORD (description of each twinning, including proposal and report) - Two reports will be generated; one of them will describe the individual twinning initiatives developed in ACCORD and a second one issued later on will summarize the overall experience with twinnings during the Joint Action.
- Deliverable 10: Guidelines for Twinning Projects on organ donation and transplantation practices in the European Union - Report providing recommendations for further twinning initiatives to be developed in the area of organ donation and transplantation beyond the project lifetime
Associated Partners:
- BULGARIA - Bulgarian Executive Transplant Agency (BEAT).
- CYPRUS - Ministry of Health.
- CZECH REPUBLIC - Czech Transplantation Center (KST).
- FRANCE - Agence de la Biomédecine (ABM).
- HUNGARY - Hungarian National Blood Transufion Service (HNBTS).
- ITALY - Centro Nazionali Trapianti (CNT).
- LITHUANIA - National Transplant Bureau (NTB).
- MALTA - DG Health Care Services within the Ministry for health, the Elderly and Community Care (MHEC).
- THE NETHERLANDS - Nederlandse Transplantatie Stichting- Dutch Transplantation Foundation (DTF).
Collaborating Partners:
- Oganisation des Établissments de Soins, Belgium.